Conventional Insecticides –
The Killer Chemicals

Conventional insecticides are among the most popular chemical control agents because they are readily available, rapid acting, and highly reliable. A single application may control several different pest species and usually forms a persistent residue that continues to kill insects for hours or even days after application. Because of their convenience and effectiveness, insecticides quickly became standard practice for pest control during the 1960’s and 1970’s. Overuse, misuse, and abuse of these chemicals have led to widespread criticism of chemical control and, in a few cases, resulted in long-term environmental consequences.
More about Toxicity The effectiveness of an insecticide usually depends on when and where the pest encounters it. Most insecticides are absorbed directly through an insect’s exoskeleton. These compounds are known as contact poisons because they are effective “on contact”. Other insecticides act as fumigants. They are released in the vapor state (as gases) and enter the insect’s body through its tracheal system. Fumigants are most effective when they are used in an enclosed area such as a greenhouse, a warehouse, or a grain bin. Still other compounds must be ingested before they have an effect. These are known as stomach poisons. They often work more slowly than fumigants or contact poisons, but they are still useful for certain types of pest control in homes and businesses.
Major Classes of
Conventional Insecticides Systemic insecticides are a special type of stomach poison. These compounds are absorbed by the tissues of a plant (or animal) without ill effects. Insect pests ingest the insecticide when they feed on the treated organism. Systemic insecticides are sometimes included in the diets of domestic animals to protect them from internal parasites (e.g., cattle grubs and other bot flies). Plant systemics can be incorporated into the soil around ornamentals or bedding plants. The insecticides are absorbed by the roots and translocated to leaves, stems, and flowers. If the insect that feeds on a treated plant doesn’t acquire a lethal dose of insecticide, it may at least be deterred from further feeding. Although systemic insecticides are commonly applied to horticultural plantings, they are not as useful for many food crops because the insecticide remains in the food after harvest.
Examples of
Common Adjuvants Insecticides contain one or more active ingredients that serve as toxicants (poisons). In their purest form (technical grade), these chemicals may be too toxic, too unstable, or too volatile to be handled or applied safely. Therefore, technical grade insecticide is always mixed with other compounds, known as adjuvants, in order to improve the performance, safety, or handling characteristics of a commercial product. These mixtures (technical grade insecticide plus adjuvants) are known as formulations. Almost anything could be an adjuvant: pumice, ground walnut shells, buffalo gourd root powder, vegetable oil, etc. These compounds are usually listed on the label as “inert ingredients”, but they are certainly not inactive. Many adjuvants are proprietary products, protected by patents and closely guarded as industrial secrets. They may represent 90-95% of the total volume of a commercial formulation.
An insecticide can be manufactured in a variety of formulations, each tailored for specific applications. Some of the more common formulations include:
 Granules (G) or pellets (P) — Coarse particles (e.g., clay, ground corn cobs, or walnut shells) can serve as a carrier for certain types of insecticides. The toxicant slowly leaches out of the carrier, minimizing its movement within the ecosystem and maximizing its active life (persistence). Granular formulations are commonly used for controlling soil-dwelling insects and in systemic insecticides (see above) that are applied around the base of plants. Since granular formulations do not blow or drift, they are considered relatively safe from the standpoint of accidental human exposure.
Granules (G) or pellets (P) — Coarse particles (e.g., clay, ground corn cobs, or walnut shells) can serve as a carrier for certain types of insecticides. The toxicant slowly leaches out of the carrier, minimizing its movement within the ecosystem and maximizing its active life (persistence). Granular formulations are commonly used for controlling soil-dwelling insects and in systemic insecticides (see above) that are applied around the base of plants. Since granular formulations do not blow or drift, they are considered relatively safe from the standpoint of accidental human exposure.
Dusts (D) — These dry powders are usually formulated with inert particles of ash, chalk, talc, or clay. They are designed to be sprinkled or blown onto target surfaces. The powder sticks to feet, legs, and other body parts of passing insects, and the toxicant is eventually ingested when the insect cleans itself. Insecticides that act as stomach poisons are often formulated as dusts. These formulations are not always suitable for outdoor applications because they have a tendency to drift (blow away with the wind).
 Soluble powders (SP) or wettable powders (WP) — Dry formulations that are mixed in water to form homogeneous spray solutions. A soluble powder dissolves completely in water, whereas a wettable powder contains an emulsifier that produces a uniform suspension (colloid). Many foliar insecticides are formulated as wettable (or soluble) powders because they are easy to transport and generally have a long shelf life.
Soluble powders (SP) or wettable powders (WP) — Dry formulations that are mixed in water to form homogeneous spray solutions. A soluble powder dissolves completely in water, whereas a wettable powder contains an emulsifier that produces a uniform suspension (colloid). Many foliar insecticides are formulated as wettable (or soluble) powders because they are easy to transport and generally have a long shelf life.
 Emulsifiable concentrates (EC) — These liquid formulations contain the toxicant(s) and an emulsifier dissolved in organic solvent. The concentrate is diluted with a large volume of water to produce the final spray mixture. Some foliar insecticides are formulated as emulsifiable concentrates. Unlike most wettable powders, they do not leave a visible residue on fruits and vegetables. Sensitive plants, however, may be injured by organic solvents in the mixture. Emulsifiable concentrates are also commonly used in sprays for urban and industrial pests.
Emulsifiable concentrates (EC) — These liquid formulations contain the toxicant(s) and an emulsifier dissolved in organic solvent. The concentrate is diluted with a large volume of water to produce the final spray mixture. Some foliar insecticides are formulated as emulsifiable concentrates. Unlike most wettable powders, they do not leave a visible residue on fruits and vegetables. Sensitive plants, however, may be injured by organic solvents in the mixture. Emulsifiable concentrates are also commonly used in sprays for urban and industrial pests.
Aerosols (A) — Insecticides that are formulated together with a solvent may be pre-packaged in pressurized spray cans or sold unpressurized for use in special fogging machines. Spray cans are relatively expensive (per pound of active ingredient) but they are convenient, easy to store, and have a long shelf life. Commercial foggers are typically used indoors (e.g., greenhouses and warehouses) or for control of biting flies in community-wide pest control operations.
 Ultralow-Volume Concentrates (ULV) — These highly concentrated formulations (more than 8 pounds of active ingredient per gallon) are designed to be used in specialized spray equipment that atomizes the concentrate droplets. ULV spray equipment is used by most aerial applicators (airplane sprayers) who treat forested lands or large agricultural acreages.
Ultralow-Volume Concentrates (ULV) — These highly concentrated formulations (more than 8 pounds of active ingredient per gallon) are designed to be used in specialized spray equipment that atomizes the concentrate droplets. ULV spray equipment is used by most aerial applicators (airplane sprayers) who treat forested lands or large agricultural acreages.
More about
Labels and Rates Despite their many advantages, conventional insecticides are not ideal pest control agents. Indeed, one of their greatest strengths, broad-spectrum activity, is also one of their greatest weaknesses. While it is certainly an advantage to control multiple pest species with a single chemical treatment, the non-specificity of most conventional insecticides poses a serious threat to non-target organisms in the environment. High mortality among natural enemies can have an enduring impact on the ecological balance of any community. In the absence of biocontrol agents, more insecticide applications may be the only recourse available to stop pest resurgence. Once we step onto this “insecticide treadmill”, it can be very difficult to get off.
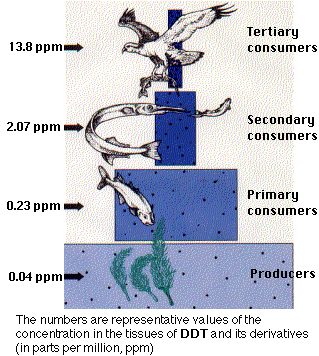
The non-target effects of insecticides are not limited to natural enemies. Most of the organochlorine compounds (e.g., DDT) have been banned from use in the United States because of their indirect effects on reproduction in birds of prey (e.g., eagles, ospreys, condors, etc.). These environmentally stable compounds are highly soluble in lipids, making it possible for them to accumulate in the body fat of non-target organisms. Predators, particularly those at the top of a food chain, amass pesticide concentrations many times greater than anywhere else in the environment. This process, known as bioaccumulation (or biomagnification), was responsible for high levels of DDT and related compounds in birds of prey during the 1960’s and 1970’s. These pesticides did not injure the birds themselves, but caused thinning of their egg shells and high rates of breakage during incubation. Most organochlorine insecticides were banned during the 1970’s and 1980’s and many of the threatened bird species are now recovering from the brink of extinction.
 In some cases, sub-lethal concentrations of an insecticide can stimulate rather than suppress the growth of a pest population. This phenomenon, known as hormoligosis, has been observed in a number of pest species, including twospotted spider mites (Tetranychus urticae), western corn rootworms (Diabrotica virgifera), and brown planthoppers (Nilaparvata lugens). Low doses of pesticide seem to improve the nutritional quality of host plants, thereby increasing a pest’s reproductive potential or decreasing its time of development.
In some cases, sub-lethal concentrations of an insecticide can stimulate rather than suppress the growth of a pest population. This phenomenon, known as hormoligosis, has been observed in a number of pest species, including twospotted spider mites (Tetranychus urticae), western corn rootworms (Diabrotica virgifera), and brown planthoppers (Nilaparvata lugens). Low doses of pesticide seem to improve the nutritional quality of host plants, thereby increasing a pest’s reproductive potential or decreasing its time of development.
And finally, there is the problem of resistance to insecticides. Insects are among the most adaptable organisms on the face of the earth. For the past 400 million years they have managed to survive by adjusting to changes in their environment, so it should come as no surprise that they can also adapt to chemical pesticides. Resistance has increased exponentially since the the late 1940’s, and today, there are over 500 pest species that exhibit some level of resistance to at least one type of insecticide.
Insects may become resistant to insecticides in several ways. Biochemical resistance usually involves changes in the metabolic pathways that insects normally use to break down plant defenses and other environmental toxins. This detoxication is facilitated by enzymes (esterases, hydrolases, transferases, and oxidases) that change the chemical structure of toxicants before they cause physical harm. Physiological resistance involves functional changes in basic life processes that alter the way toxicants interact with the body. Some German cockroaches (Blatella germanica), for example, have become resistant to carbaryl (a carbamate insecticide) as a result of genetic changes in the permeability of their cuticle. Behavioral resistance may occur as a result of any innate change in behavior that reduces an insect’s probability of encountering a toxicant. In some parts of Panama, the mosquitoes that vector malaria (Anopheles albimanus) have become hypersensitive to certain insecticides. They manage to escape lethal doses of insecticide by avoiding enclosed areas and refusing to land on treated surfaces.
When an insect population becomes resistant to one insecticide, it may also become less susceptible to other toxicants in the same chemical family. This phenomenon, known as class resistance, is a common problem in all major groups of insecticides (organochlorines, organophosphates, carbamates, and synthetic pyrethroids). When an insecticide loses its effectiveness because of pest resistance, users typically replace it with another compound from a different chemical group. But resistance to one group of compounds does not prevent subsequent development of resistance to compounds in other chemical groups. More about
Resistance Over the years, some pests have developed multiple resistance to nearly every insecticide that has ever been used to fight them. Colorado potato beetles, Leptinotarsa decemlineata, on Long Island, N.Y. are probably the best example of a population that has developed multiple resistance. It may never be possible to prevent insects from developing resistance, but there are ways to manage these chemicals that will prolong their useful life.

