All category I and II pesticides (as well as some category III pesticides) are designated as “restricted use” chemicals. These compounds may only be sold to (and used by) people who have been “certified” by an agency of their state government and received a pesticide applicator’s license. This certification process usually involves special training and a written examination. Only “general use” chemicals may be purchased by the public without a pesticide applicator’s license.
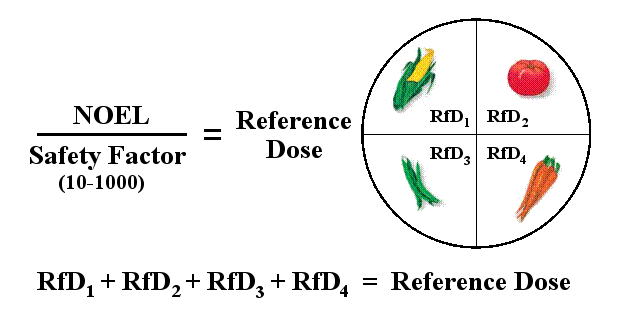
The EPA regulates the safety of our food supply by limiting the residual amount of pesticide (residue) that legally may be present on each agricultural product at harvest. This maximum limit, called a tolerance, is established through the above process of risk assessment. Using the dose-response curves found during animal studies of acute and chronic exposure to a pesticide, the EPA determines a level of exposure that should have no observable effect in humans (NOEL = No Observable Effect Level). To insure a significant margin of safety, the NOEL value is further divided by an “uncertainty factor” (usually about 100) to give a Reference Dose (RfD). The uncertainty factor is usually higher for pesticides that are more hazardous or may be applied to foods that are consumed in greater quantities by children, older adults, or other “at-risk” populations. For each agricultural product that will be treated with the insecticide, EPA sets a tolerance (well below the reference dose) based on an ongoing “market basket survey” by the FDA that estimates the types and amounts of foods consumed in the typical American diet. This process insures that no matter what combination of treated produce is consumed, the intake of a pesticide will never exceed its reference dose.
 Once a tolerance has been issued, the chemical company must submit a label for approval both by the EPA and by state governments where the pesticide will be sold. The label specifies the commodities on which the pesticide may be used and limits the application rate so residues will not exceed the tolerance.
Once a tolerance has been issued, the chemical company must submit a label for approval both by the EPA and by state governments where the pesticide will be sold. The label specifies the commodities on which the pesticide may be used and limits the application rate so residues will not exceed the tolerance.
More about
Labels and Rates Chemical companies must spend millions of dollars in research and development costs to shepherd each new pesticide through the regulatory jungle. In order for companies to make any money, they must recoup their expenses and return a profit to shareholders before the chemical’s patent expires in 17 years. As a result, there is no incentive to develop products for minor crops or niche markets because the financial return is just too small. If a pesticide cannot be used on a major crop like corn, wheat, soybeans, or cotton, it will probably never become registered. Two legislative provisions help to counteract this problem:
- Under authority of Section 24(c) of the Federal Insecticide, Fungicide, and Rodenticide Act, state governments may register additional uses of a federally registered pesticide to meet a “special local need”. Such state registrations cut the time, expense, and red tape needed to get a pesticide approved for a regional crop or a local pest problem. One caveat, however, is that the pesticide must already have a federal label for some other use.
- Interregional Research Project No. 4 (the IR-4 Project) is a “minor use pesticides” program. It is a partnership, established in 1963, between the USDA and the land grant university system to help farmers, agriculture scientists, and Extension personnel conduct research and obtain tolerances for specific pesticide uses on specialty food crops (such as fruits, vegetables, and herbs) and other minor uses (such as floral, nursery, or turf crops).
Although each “minor crop” is only a small percentage of U.S. agriculture, as a group they represent about 40% of the agricultural acreage and more than $40 billion in annual farm revenue. That’s no small potatoes!

 Once a tolerance has been issued, the chemical company must submit a label for approval both by the EPA and by state governments where the pesticide will be sold. The label specifies the commodities on which the pesticide may be used and limits the application rate so residues will not exceed the tolerance.
Once a tolerance has been issued, the chemical company must submit a label for approval both by the EPA and by state governments where the pesticide will be sold. The label specifies the commodities on which the pesticide may be used and limits the application rate so residues will not exceed the tolerance.