Oils and Soaps
Water emulsions of petroleum distillates are commonly known as crop oils or dormant oils. They act by coating and suffocating small insects or their eggs. Insecticidal soaps are derived from animal or vegetable oils. They have been used as contact insecticides since 1787. Oils and soaps are most effective against scale insects, aphids, whiteflies, and phytophagous mites.
Botanical extracts
Plant extracts have been used to kill insects since ancient times. These compounds represent a wide range of chemical structures and activities.
Nicotine
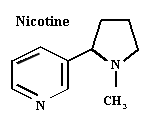 Source: Tobacco (Nicotiana spp.) — Solanaceae. Sold commercially as a fumigant (nicotine) or as a dust (nicotine sulfate).
Source: Tobacco (Nicotiana spp.) — Solanaceae. Sold commercially as a fumigant (nicotine) or as a dust (nicotine sulfate).
Activity: Mimics acetylcholine in the nerve synapse, causing tremors, loss of coordination, and eventually death.
Sabadilla
Source: Schoenocaulon officinale — Liliaceae. Grown commercially in Venezuela.
Activity: Contains alkaloids (primarily cevadine and veratridine) that act as nerve poisons.
Rotenone
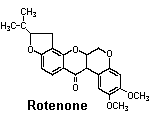 Source: Tropical legumes — Leguminaceae. Derris spp. grown in Malaysia and the East Indies and Lonchocarpus spp. grown in South America
Source: Tropical legumes — Leguminaceae. Derris spp. grown in Malaysia and the East Indies and Lonchocarpus spp. grown in South America
Activity: Rotenone is a metabolic poison. It inhibits electron transport during aerobic respiration by blocking the action of an essential enzyme, NADH2dehydrogenase. Rotenone is extremely toxic to fish.
Ryanodine
Source: Ryania speciosa — Flacourtaceae.
Activity: Ryanodine acts as a muscular poison by blocking the conversion of ADP to ATP in striated muscles.
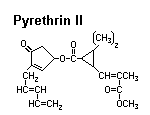
Pyrethrum
Source: Chrysanthemum spp. — Asteraceae. Most of the world’s supply is derived from two species grown commercially in Kenya.
Activity: Pyrethrums change the permeability of sodium channels in the nerve axon. This typically results in excitation, lack of coordination, and paralysis. Since the effects of pyrethrum poisoning are often reversible, commercial insecticides usually contain a synergist, such as piperonyl butoxide, that blocks the insect’s detoxication pathway.
Organochlorines
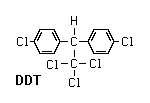 These were the first synthetic organic insecticides ever discovered. The group includes DDT and its relatives (e.g., methoxychlor and kelthane), lindane, toxaphene, and cyclodiene insecticides (e.g., aldrin, dieldrin, endrin, mirex, chlordane, heptachlor, and endosulfan). Most organochlorines (they are also known as chlorinated hydrocarbons) are relatively stable compounds that act as nerve poisons. They are several thousand times more soluble in fat (lipid) than in water. This means they tend to accumulate in fatty tissues and concentrate in organisms at the top of a community’s food chain (bioaccumulation). Most uses of organochlorines have been banned or discontinued in the United States because of environmental problems associated with their long persistence and bioaccumulation. Lindane is still used as a seed dressing and sold by prescription for control of human lice; endosulfan and kelthane are still used for agricultural pest control.
These were the first synthetic organic insecticides ever discovered. The group includes DDT and its relatives (e.g., methoxychlor and kelthane), lindane, toxaphene, and cyclodiene insecticides (e.g., aldrin, dieldrin, endrin, mirex, chlordane, heptachlor, and endosulfan). Most organochlorines (they are also known as chlorinated hydrocarbons) are relatively stable compounds that act as nerve poisons. They are several thousand times more soluble in fat (lipid) than in water. This means they tend to accumulate in fatty tissues and concentrate in organisms at the top of a community’s food chain (bioaccumulation). Most uses of organochlorines have been banned or discontinued in the United States because of environmental problems associated with their long persistence and bioaccumulation. Lindane is still used as a seed dressing and sold by prescription for control of human lice; endosulfan and kelthane are still used for agricultural pest control.
Organophosphates
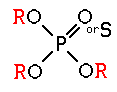 These compounds were first discovered by German scientists in the late 1930’s as a by-product of nerve gas research. They are much less persistent than the organochlorines and do not accumulate in fatty tissues. The group includes many general purpose insecticides with a wide range of mammalian toxicity (e.g. malathion, parathion, diazinon, chlorpyrifos, azinphosmethyl, acephate, phorate, and phosmet) as well as compounds that work as fumigants (e.g., DDVP) and as systemics (e.g. dimethoate, disulfoton, demeton, and ronnel). All of the organophosphates are nerve poisons. They block the active site of an enzyme (acetylcholinesterase) that breaks down and removes a neurotransmitter substance (acetylcholine) from the nerve synapse. The excessive build up of acetylcholine results in symptoms of hyperactivity, including tremors, convulsions, and eventually death.
These compounds were first discovered by German scientists in the late 1930’s as a by-product of nerve gas research. They are much less persistent than the organochlorines and do not accumulate in fatty tissues. The group includes many general purpose insecticides with a wide range of mammalian toxicity (e.g. malathion, parathion, diazinon, chlorpyrifos, azinphosmethyl, acephate, phorate, and phosmet) as well as compounds that work as fumigants (e.g., DDVP) and as systemics (e.g. dimethoate, disulfoton, demeton, and ronnel). All of the organophosphates are nerve poisons. They block the active site of an enzyme (acetylcholinesterase) that breaks down and removes a neurotransmitter substance (acetylcholine) from the nerve synapse. The excessive build up of acetylcholine results in symptoms of hyperactivity, including tremors, convulsions, and eventually death.
Carbamates
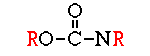 First developed in the early 1950’s, this large family of insecticides is comparable in many respects to the organophosphates, sharing a common mode of action, biodegradability, low solubility in fat, and a wide range of mammalian toxicity. Carbaryl (Sevin) is probably the best known and most widely used carbamate; it also the least toxic to humans. Other carbamates include carbofuran, propoxur, methomyl, bendiocarb, formetanate, oxamyl, and aldicarb (the last three are plant systemics). One advantage carbamates have over organophosphates is reversibility of their inhibitory reaction with acetylcholinesterase. Chronic exposure to carbamates, therefore, is less likely to cause illness than chronic exposure to organophosphates.
First developed in the early 1950’s, this large family of insecticides is comparable in many respects to the organophosphates, sharing a common mode of action, biodegradability, low solubility in fat, and a wide range of mammalian toxicity. Carbaryl (Sevin) is probably the best known and most widely used carbamate; it also the least toxic to humans. Other carbamates include carbofuran, propoxur, methomyl, bendiocarb, formetanate, oxamyl, and aldicarb (the last three are plant systemics). One advantage carbamates have over organophosphates is reversibility of their inhibitory reaction with acetylcholinesterase. Chronic exposure to carbamates, therefore, is less likely to cause illness than chronic exposure to organophosphates.
Synthetic Pyrethroids
Modeled after the natural product found in chrysanthemums, these insecticides are highly toxic to insects yet relatively safe to humans. Natural pyrethrum is nearly useless outdoors because it breaks down rapidly in sunlight. The first light-stable analogue was synthesized in 1967 (resmethrin), and by 1976 permethrin and fenvalerate were commercially available as agricultural insecticides. All of these compounds act as nerve poisons; they appear to disrupt sodium transport in axons in much the same way as some of the organochlorines.
Foramidines
These compounds (e.g., chlordimeform and amitraz) were first developed for commercial use in the 1970’s. They probably mimic the activity of octopamine, a neurotransmitter substance found in the insect’s central nervous system. Since their mode of action is different from other insecticides, they have been found useful for controlling some of the pests that have become resistant to organophosphates or carbamates.
Organosulfurs and Organotins
These compounds (e.g., aramite, tetradifon, cyhexatin, and hexakis) are much more effective against phytophagous mites than they are against insects. As a result, they are commonly used as miticides in greenhouses, nurseries, and orchards. Organosulfurs are especially useful because they have ovicidal activity (i.e. they kill the egg stage).
Avermectins
The chemicals in this new class of compounds have been isolated from a soil-dwelling fungus, Streptomyces avermitilis. These pesticides (e.g., avermectin, abamectin, and ivermectin), cause paralysis and death by inhibiting transmission of nerve impulses across the neuromuscular synapse. The avermectins have a rather narrow activity spectrum: they affect only insects, phytophagous mites, and certain plant-parasitic nematodes.
Neonicotinoids
Imidacloprid is the first member of this new class of chemical insecticides. It has both systemic and contact activity against a wide variety of sucking insects like aphids, leafhoppers, and whiteflies. Imidacloprid’s mode of action appears to be similar to that of nicotine: it mimics the action of acetylcholine in the nerve synapse, causing tremors, loss of coordination, and eventual death.
 There are more than 400 specific chemical compounds that are currently registered for use in the United States as insecticides. They represent a wide range of chemical structures, toxicity, and physical properties. Some are general purpose insecticides, while others have very specific and limited uses. In addition, there are many other compounds that have been used in the past but are no longer available because of high toxicity, long persistence, or low reliability. The following list, organized by chemical families, includes some of the more important insecticides — both past and present.
There are more than 400 specific chemical compounds that are currently registered for use in the United States as insecticides. They represent a wide range of chemical structures, toxicity, and physical properties. Some are general purpose insecticides, while others have very specific and limited uses. In addition, there are many other compounds that have been used in the past but are no longer available because of high toxicity, long persistence, or low reliability. The following list, organized by chemical families, includes some of the more important insecticides — both past and present. Arsenic and fluoride compounds were the mainstay of chemical pest control from the mid 1800’s to the mid 1900’s. These materials [e.g., lead arsenate, arsenic trioxide, and copper acetoarsenate (Paris green)], are persistent in the environment and highly toxic to all forms of animal life. Most inorganic compounds were phased out of use after World War II. They were replaced by synthetic organic compounds that were more effective and less hazardous to humans and the environment. Sodium fluoride (NaF) and cryolite (sodium fluoroaluminate, Na3AlF6) are stomach poisons that still have commercial applications. Finely ground sulfur is also used as both a fungicide and a miticide.
Arsenic and fluoride compounds were the mainstay of chemical pest control from the mid 1800’s to the mid 1900’s. These materials [e.g., lead arsenate, arsenic trioxide, and copper acetoarsenate (Paris green)], are persistent in the environment and highly toxic to all forms of animal life. Most inorganic compounds were phased out of use after World War II. They were replaced by synthetic organic compounds that were more effective and less hazardous to humans and the environment. Sodium fluoride (NaF) and cryolite (sodium fluoroaluminate, Na3AlF6) are stomach poisons that still have commercial applications. Finely ground sulfur is also used as both a fungicide and a miticide.
 Source: Tobacco (Nicotiana spp.) — Solanaceae. Sold commercially as a fumigant (nicotine) or as a dust (nicotine sulfate).
Source: Tobacco (Nicotiana spp.) — Solanaceae. Sold commercially as a fumigant (nicotine) or as a dust (nicotine sulfate). Source: Tropical legumes — Leguminaceae. Derris spp. grown in Malaysia and the East Indies and Lonchocarpus spp. grown in South America
Source: Tropical legumes — Leguminaceae. Derris spp. grown in Malaysia and the East Indies and Lonchocarpus spp. grown in South America
 These were the first synthetic organic insecticides ever discovered. The group includes DDT and its relatives (e.g., methoxychlor and kelthane), lindane, toxaphene, and cyclodiene insecticides (e.g., aldrin, dieldrin, endrin, mirex, chlordane, heptachlor, and endosulfan). Most organochlorines (they are also known as chlorinated hydrocarbons) are relatively stable compounds that act as nerve poisons. They are several thousand times more soluble in fat (lipid) than in water. This means they tend to accumulate in fatty tissues and concentrate in organisms at the top of a community’s food chain (bioaccumulation). Most uses of organochlorines have been banned or discontinued in the United States because of environmental problems associated with their long persistence and bioaccumulation. Lindane is still used as a seed dressing and sold by prescription for control of human lice; endosulfan and kelthane are still used for agricultural pest control.
These were the first synthetic organic insecticides ever discovered. The group includes DDT and its relatives (e.g., methoxychlor and kelthane), lindane, toxaphene, and cyclodiene insecticides (e.g., aldrin, dieldrin, endrin, mirex, chlordane, heptachlor, and endosulfan). Most organochlorines (they are also known as chlorinated hydrocarbons) are relatively stable compounds that act as nerve poisons. They are several thousand times more soluble in fat (lipid) than in water. This means they tend to accumulate in fatty tissues and concentrate in organisms at the top of a community’s food chain (bioaccumulation). Most uses of organochlorines have been banned or discontinued in the United States because of environmental problems associated with their long persistence and bioaccumulation. Lindane is still used as a seed dressing and sold by prescription for control of human lice; endosulfan and kelthane are still used for agricultural pest control. These compounds were first discovered by German scientists in the late 1930’s as a by-product of nerve gas research. They are much less persistent than the organochlorines and do not accumulate in fatty tissues. The group includes many general purpose insecticides with a wide range of mammalian toxicity (e.g. malathion, parathion, diazinon, chlorpyrifos, azinphosmethyl, acephate, phorate, and phosmet) as well as compounds that work as fumigants (e.g., DDVP) and as systemics (e.g. dimethoate, disulfoton, demeton, and ronnel). All of the organophosphates are nerve poisons. They block the active site of an enzyme (acetylcholinesterase) that breaks down and removes a neurotransmitter substance (acetylcholine) from the nerve synapse. The excessive build up of acetylcholine results in symptoms of hyperactivity, including tremors, convulsions, and eventually death.
These compounds were first discovered by German scientists in the late 1930’s as a by-product of nerve gas research. They are much less persistent than the organochlorines and do not accumulate in fatty tissues. The group includes many general purpose insecticides with a wide range of mammalian toxicity (e.g. malathion, parathion, diazinon, chlorpyrifos, azinphosmethyl, acephate, phorate, and phosmet) as well as compounds that work as fumigants (e.g., DDVP) and as systemics (e.g. dimethoate, disulfoton, demeton, and ronnel). All of the organophosphates are nerve poisons. They block the active site of an enzyme (acetylcholinesterase) that breaks down and removes a neurotransmitter substance (acetylcholine) from the nerve synapse. The excessive build up of acetylcholine results in symptoms of hyperactivity, including tremors, convulsions, and eventually death.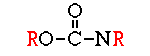 First developed in the early 1950’s, this large family of insecticides is comparable in many respects to the organophosphates, sharing a common mode of action, biodegradability, low solubility in fat, and a wide range of mammalian toxicity. Carbaryl (Sevin) is probably the best known and most widely used carbamate; it also the least toxic to humans. Other carbamates include carbofuran, propoxur, methomyl, bendiocarb, formetanate, oxamyl, and aldicarb (the last three are plant systemics). One advantage carbamates have over organophosphates is reversibility of their inhibitory reaction with acetylcholinesterase. Chronic exposure to carbamates, therefore, is less likely to cause illness than chronic exposure to organophosphates.
First developed in the early 1950’s, this large family of insecticides is comparable in many respects to the organophosphates, sharing a common mode of action, biodegradability, low solubility in fat, and a wide range of mammalian toxicity. Carbaryl (Sevin) is probably the best known and most widely used carbamate; it also the least toxic to humans. Other carbamates include carbofuran, propoxur, methomyl, bendiocarb, formetanate, oxamyl, and aldicarb (the last three are plant systemics). One advantage carbamates have over organophosphates is reversibility of their inhibitory reaction with acetylcholinesterase. Chronic exposure to carbamates, therefore, is less likely to cause illness than chronic exposure to organophosphates.